1.Research background and concept of fast charging battery
With the introduction of the “double carbon” goal, a low-carbon trend in global energy and industrial development has emerged. Consequently, utilizing clean energy to power vehicles holds great significance in reducing CO2 emissions and achieving carbon neutrality. Electric vehicles powered by lithium-ion batteries have increasingly drawn attention due to their high energy density, long cycle life, low cost, and minimal environmental pollution.
Nevertheless, despite projections that the global electric vehicle fleet will hit 230 million by 2030, both market penetration and consumer acceptance remain relatively low at present. One of the important reasons is mileage anxiety. Therefore, fast charging lithium-ion batteries have become a research hotspot in recent years.
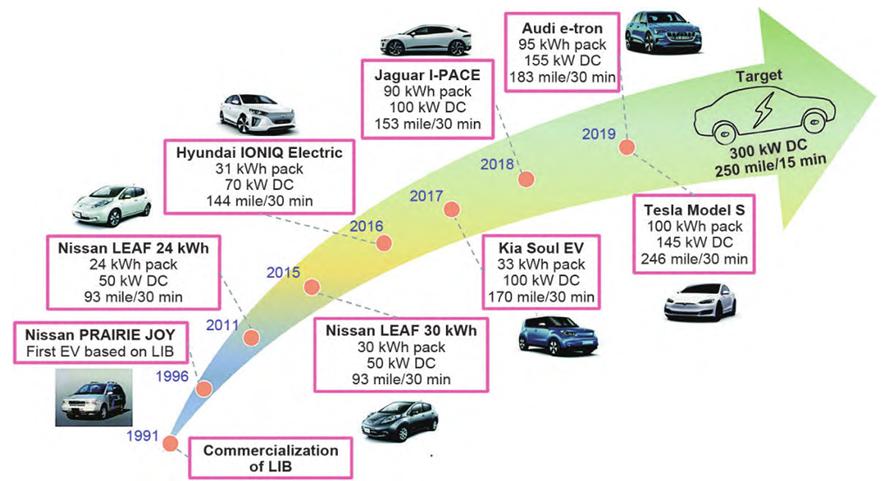
Figure-1-The-development-history-of-lithium-ion-battery-electric-vehicles-and-the-corresponding-fast-charging-capacity
As per the definition by the United States Advanced Battery Consortium (USABC), fast charging refers to achieving 80% of the battery’s state of charge (SOC) within 15 minutes, which means charging the battery pack to 80% at a rate of 4 C. Moreover, fast-charging lithium-ion batteries must be assessed on three key metrics simultaneously: 1. Charging time; 2. The specific energy obtained; 3. The number of cycles at high rates. While lithium-ion battery electric vehicles have made notable advancements in fast charging (see Figure 1), there is still a gap to bridge in order to reach the target.
2.Effect of fast charging battery on lithium ion battery
Lithium-ion batteries, often referred to as “rocking chair” batteries, operate through the shuttling of Li+ ions between the cathode and anode. A crucial element that determines the battery’s performance is the mobility of lithium ions. This encompasses the diffusion of Li+ within the electrode material, the movement of Li+ through the solid electrolyte interphase (SEI), and the migration of Li+ in the electrolyte solution. When batteries undergo rapid charging and discharging, both the electrochemical reactions and the intrinsic structure of the battery can influence the transfer of ions and electrons, which in turn affects overall performance. The influence of fast-charging technology on lithium batteries is mainly manifested in the following aspects:
1.Lithium plating: Among all factors causing performance degradation of lithium-ion batteries under fast charging, the most detrimental is lithium plating on the surface of the graphite negative electrode (see Figure 2). During charging, Li+ migrates from the positive electrode to the negative electrode and intercalates into the graphite layer. However, under fast-charging conditions, the rate of Li+ transport in the electrolyte far exceeds that of Li+ intercalation into the graphite layer. As a result, more Li+ accumulates on the negative electrode surface rather than being intercalated into the graphite atomic layer gaps. This leads to severe voltage polarization and reduces the graphite negative electrode potential to 0 V (vs. Li/Li+). The deposited lithium further reacts with the electrolyte to form an ineffective SEI layer or a “dead” lithium film isolated from the anode. The high impedance not only increases the battery’s internal resistance but also reduces its energy density, accelerating battery capacity fade.
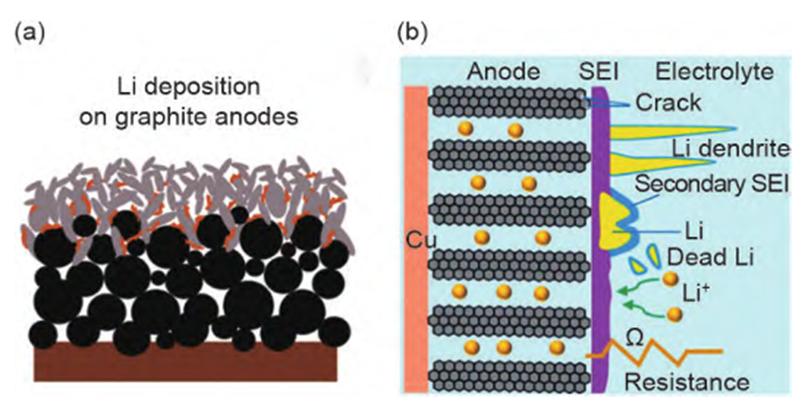
Figure-2-Graph-of-lithium-plating-on-graphite-anode-under-fast-charge-condition-and-its-degradation-mechanism
2.Mechanical effect: Mechanical degradation occurs on various scales, such as the separation of electrode particles, conductive agents, and binders, internal fractures within electrode particles, detachment of the active material from the current collector, and delamination between electrode layers. These issues primarily stem from uneven stress between components due to the lithium concentration gradient during fast charging. As fast charging continues, Li+ ions are swiftly extracted from the cathode and inserted into the anode, causing significant strain mismatches between Li+ and different parts of the electrode particles. When the energy release rate or stress intensity factor surpasses a certain threshold, cracks propagate within the particles, leading to SEI/CEI fractures. The effects of mechanical degradation on battery performance are mainly due to active material loss, lithium storage loss, and impedance increase.
3.Thermal effect: Elevated charging rates can trigger heating issues, which in turn degrade battery performance. The heightened temperature within the battery can expedite a variety of side reactions, such as material phase transitions, gas emission, binder breakdown, and metal dissolution. Specifically, lattice expansion induced by high temperatures can exacerbate volume expansion, resulting in mechanical stress and potentially particle cracking. As the temperature rises, the electrolyte may undergo thermal decomposition, with the resultant gaseous by-products further intensifying mechanical stress. Moreover, studies have shown that an increase in charging rate correlates with a decrease in the battery’s thermal stability. Thermal runaway, a common consequence, can lead to smoke, fire, and even explosions, posing a significant threat to battery safety.
4.Others: In the fast charging process of the battery, the polarization will increase significantly, which will affect the specific capacity, energy density and cycle life of the battery.
3.The main research direction of fast charging battery
To further enhance the fast-charging capabilities of batteries, researchers have achieved notable advancements across various dimensions. The optimization strategies primarily focus on the following areas:
1.The optimization of the negative electrode: For the negative electrode, ensuring rapid Li+ diffusion within the bulk phase and reducing the kinetic barrier at the electrode–electrolyte interface are crucial for achieving fast charging. If Li+ transport kinetics are insufficient for fast charging, excessive polarization can lead to lithium plating on the negative electrode, which not only shortens cycle life but also poses safety risks. Enhancing Li+ and electron transport kinetics is thus a key strategy to enable fast charging.
2.Optimization of cathode materials:Common cathode materials for lithium-ion batteries include LiCoO₂, LiFePO₄, and ternary materials (LiNiₓCoᵧAlzO₂ and LiNiₓCoᵧMnzO₂). Among these, ternary materials are known for their high specific capacity, elevated voltage platform, and robust cycle performance, making them a promising candidate for fast-charging lithium-ion batteries. However, improvements in safety are still needed.
The ideal cathode material should possess rapid Li⁺ deintercalation capabilities and maintain stable cycle performance. Current research efforts are focused on enhancing the performance of existing cathode materials through modifications and developing new cathode materials tailored for fast charging.
3.Optimization of electrolytes:The traditional lithium-ion battery electrolyte primarily consists of lithium salts and organic solvents, and its composition and solvation structure significantly influence the transport kinetics of Li⁺ in the electrolyte and the formation of SEI/CEI. During the charge-discharge process, Li⁺ not only undergoes delithiation and intercalation reactions within the electrode material but also completes the solvation and desolvation processes on the electrode surface, contributing to the formation of the SEI/CEI structure. The properties of the SEI layer can substantially impact the battery’s rate performance. Fast charging can induce side reactions that reduce the electrolyte’s stability, while internal heat generation or lithium dendrite growth can decrease electrolyte conductivity and trigger exothermic reactions. Therefore, developing electrolytes that meet the requirements for fast charging and high safety has become a research hotspot.
4.The case study of fast charging battery
1.Optimization of anode: Taking graphite anode material as an example, it is the most commonly used carbon-based anode material for lithium-ion batteries. Graphite features a layer spacing of 0.34 nm, which enables the reversible intercalation and exfoliation of Li⁺. Additionally, graphite’s potential is very close to the redox potential of lithium, allowing the battery to achieve a higher energy density. However, graphite suffers from slow lithium insertion kinetics and a low lithiation voltage (0.08 V vs. Li/Li⁺). Under high current conditions, significant polarization can push the graphite potential to the threshold of lithium metal deposition (0 V vs. Li/Li⁺), leading to lithium plating on the graphite surface.
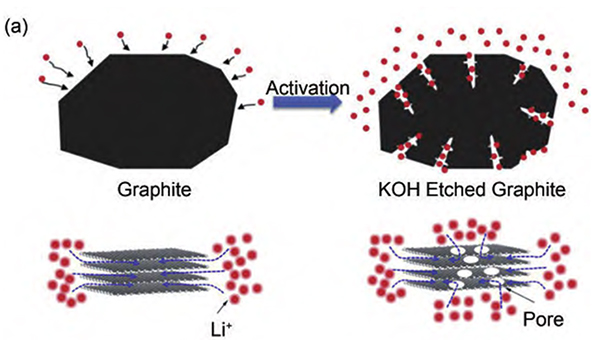
Figure-3-Schematic-diagram-of-original-graphite-and-KOH-etched-graphite
In order to solve this problem, a research team proposed to etch graphite with KOH to form holes on its surface that can reduce the diffusion distance of Li+ to improve the charge and discharge rate capability (Figure 3). The capacity retention rate of KOH-etched graphite at 3 C charge and discharge is 93%. Even at a higher cycle rate of 6 C, it can still exhibit a capacity retention rate of 74% (Figure 4).
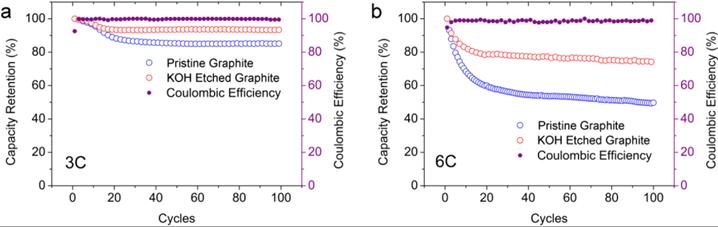
Figure-4-Comparison-of-electrochemical-performance
2.Optimization of the cathode: The use of element doping to enhance the performance of cathodes has garnered significant attention. Research indicates that element doping can alter the lattice size, expand the Li⁺ diffusion channels, mitigate strain caused by lattice volume changes during cycling, and improve mechanical stability.
A research team has successfully doped Mg and Ti into LiCoO₂ (LCO) materials. Co-doping with Mg²⁺ and Ti⁴⁺ not only enhances the rate performance and high-voltage cycle stability of LCO cathodes but also optimizes particle size distribution and reduces charge transfer resistance. This leads to an increased Li⁺ diffusion coefficient in the cathode. Specifically, the co-doped sample exhibits an initial discharge capacity of 179.6 mAh/g within a voltage range of 2.75–4.5 V at 1 C. After 100 cycles, the capacity retention rate reaches 82.6%. Additionally, the co-doped sample demonstrates superior rate performance, with a high discharge capacity of 151.4 mAh/g at 5 C.
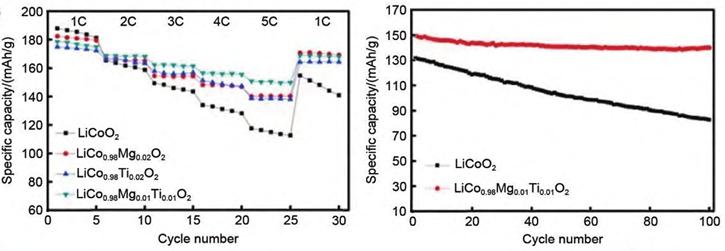
Figure-5-Electrochemical-cycling-performance-of-different-LCO-materials
3.Optimization of electrolytes: High concentration electrolytes (usually>3 mol/L) can achieve better lithium-ion batteries by adjusting the solvation structure of Li+. The evolution of the solvent structure of typical high concentration electrolytes at different lithium salt concentrations is shown in Figure 6.
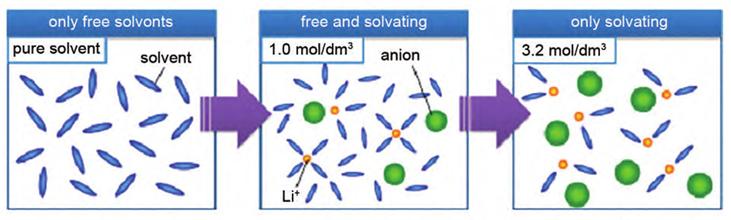
Figure-6-The-schematic-diagram-of-coordination-structure-in-low-concentration-electrolyte-and-high-concentration-electrolyte
In order to achieve the purpose of fast charging, a local high-concentration electrolyte composed of LiFSI, bis(2,2,2-trifluoroethyl) ether (BTFE) and dimethoxyethane (DME) was proposed by the scientific research team. In this system, the free solvent molecules disappear and contact ion pairs (CIPs) and aggregates (AGGs) are formed. The anions driven by CIPs and AGGs achieve their decomposition voltage priority decomposition earlier than the solvent molecules, and a uniform and strong LiF-rich SEI can be formed on the surface of graphite, which can significantly inhibit the co-embedding of solvents in graphite. Highly reversible Li+ insertion/extraction is achieved. As shown in Figure 7, the C/Li battery exhibits the potential for fast charging (a high capacity of 220 mAh/g at 4 C) and excellent cycle stability (85.5 % capacity retention after 200 cycles at 4 C).
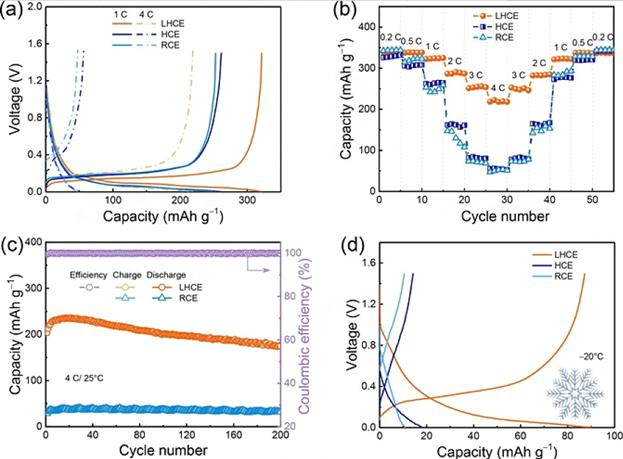
Figure-7-Comparison-of-electrochemical-properties-of-different-electrolytes
About NEWARE:
Neware was founded in 1998. We are trusted by ATL, BYD, CATL, Tesla, Apple, HUAWEI, SolarEdge, etc. We provide battery testing solutions for testing battery cell, module, pack, supercapacitor, BESS, etc. If you want to do capacity, cycle life, pulse, DCIR, GITT, HPPC, or EV driving simulation test, please feel free to contact us.
