All-solid-state sodium-ion batteries operating at room temperature based on NASICON-type NaTi2(PO4)3 cathode and ceramic NASICON solid electrolyte: A complete in situ synchrotron X-ray study
Highlights
•NASICON-type NaTi2(PO4)3/C is developed as cathode for sodium-ion battery.
•Solid-state electrolyte Na3.16Zr1.84Y0.16Si2PO12 is prepared.
•Prepared All-solid-state sodium-ion battery works at room temperature.
•The in situ synchrotron X-ray study shows the bi-phasic reaction of cathode.
•Sequential Rietveld refinements are analyzed to understand the phase evolution.
Abstract
All-solid-state sodium-ion batteries that work at ambient temperature are a potential approach for large-scale energy storage systems. Nowadays, ceramic solid electrolytes are gaining attention because of their good ionic conductivity and excellent mechanical and chemical stabilities. Furthermore, a good interface between electrode and solid electrolyte is also required to achieve successful cell performances. In this work, sintered ceramic layer electrolyte Na3.16Zr1.84Y0.16Si2PO12, with high ionic conductivity (0.202 mS/cm at room temperature), are prepared by using uniaxial pressing followed by a sintering process. The conductive carbon coated NASICON material (NaTi2(PO4)3/C) exhibits, as cathode material, enhanced rate capability and stability for sodium ion batteries for high carbon (18.95 %) coated sample. At C/10, the optimized cathode (with higher carbon content) achieves a remarkable initial discharge capacity of 107.3 mAh/g (reversible capacity of 101.4 mAh/g), a sufficient rate capability up to a rate of 10C, and a long cycle life (capacity retention of 58% after 950 cycles). The one-stage reversible biphasic reaction mechanism and potential-dependent structure–property of NaTi2(PO4)3 can be explained by employing in situ X-ray synchrotron method. Sequential Rietveld refinements of the in situ data show the evolution of the Na-poor NaTi2(PO4)3 and Na-rich Na3Ti2(PO4)3 phase fractions (wt%), unit cell characteristics, and unit cell volume. The design of an all-solid-state sodium ion half-cell with a NaTi2(PO4)3/C cathode and a Na3.16Zr1.84Y0.16Si2PO12 solid-state electrolyte interface results in stable capacity of 83.6 mAh/g at C/10 and excellent reversible capacity at high C-rate. The results show that sintered NASICON-based electrolytes can significantly contribute for the fabrication of all-solid-state sodium-ion battery due to the superior conductivity and stability.
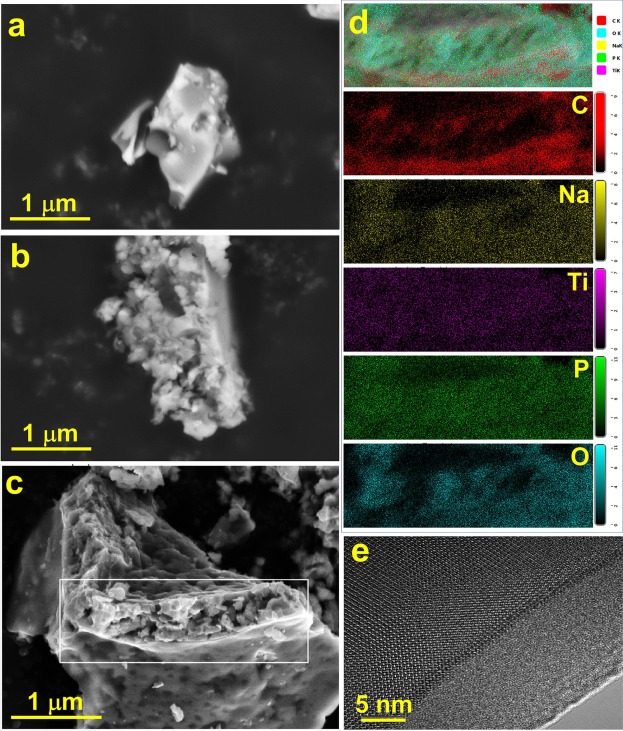
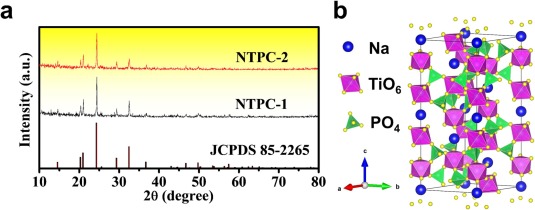
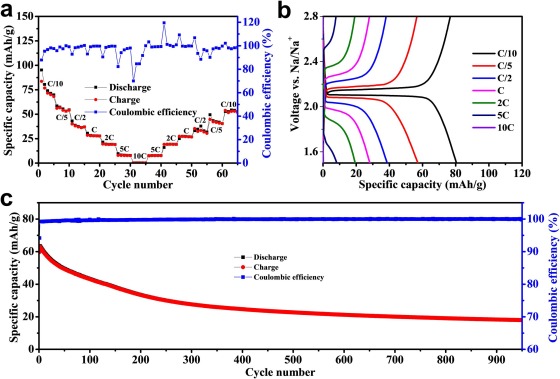
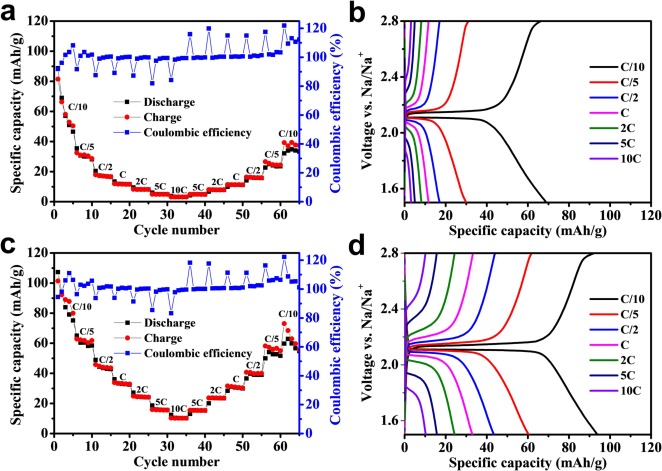
Conclusions
In summary, a solid-state technique was used to create a series of carbon-coated NaTi2(PO4)3 samples for use as electrode materials in a sodium-ion battery. The carbon covering shell significantly increases the electrochemical performance of the material. The carbon coating has no influence on the samples’ fundamental atomic structure, which are all pure phase NaTi2(PO4)3. It also has high cycle performance and low polarization. By speeding the movement of electrons and sodium ions, the carbon coating technique increases the sodium storage performance of NaTi2(PO4)3. This phenomenon will persist until all solid-state cells are made. All-solid-state cells constructed using Na3.16Zr1.84Y0.16Si2PO12 as the solid electrolyte and NaTi2(PO4)3/C as cathode exhibit, at room temperature, excellent Coulombic efficiency, high specific capacity, and reasonable cycle stability. This is probably due to (i) low total ionic conductivity of the electrolyte (ii) the effective ion exchange in the interface and (iii) to a minimized stress caused by the less volume change of electroactive material.
Through the use of operando PXRD, it is demonstrated for the first time that the reversible structural evolution of NaTi2(PO4)3 ⇋ Na3Ti2(PO4)3 during discharge and charge follows typical two-phase reaction path with small solid solution behavior in both phases close to the fully discharged and charged states, respectively. In addition, the Na-poor NaTi2(PO4)3 initially forms as tiny nanocrystals, which can form in large numbers but are too small to diffract X-rays. As a result, at 50 state-of-charge, the change in wt% for the two phases are neither symmetrical or linear. This work, which is based on the NASICON design, effectively presents a proof of concept for a prospective all-solid-state Na-ion battery as a high-performance energy storage device.
More: https://www.sciencedirect.com/science/article/pii/S1385894723032400
About NEWARE:
Neware was founded in 1998. We are trusted by ATL, BYD, CATL, Tesla, Apple, HUAWEI, SolarEdge, etc. We provide battery testing solutions for testing battery cell, module, pack, supercapacitor, BESS, etc. If you want to do capacity, cycle life, pulse, DCIR, GITT, HPPC, or EV driving simulation test, please feel free to contact us.
