Abstract
Energy production and storage technologies have attracted a great deal of attention for day-to-day applications. In recent decades, advances in lithium-ion battery (LIB) technology have improved living conditions around the globe. LIBs are used in most mobile electronic devices as well as in zero-emission electronic vehicles. However, there are increasing concerns regarding load leveling of renewable energy sources and the smart grid as well as the sustainability of lithium sources due to their limited availability and consequent expected price increase. Therefore, whether LIBs alone can satisfy the rising demand for small- and/or mid-to-large-format energy storage applications remains unclear. To mitigate these issues, recent research has focused on alternative energy storage systems. Sodium-ion batteries (SIBs) are considered as the best candidate power sources because sodium is widely available and exhibits similar chemistry to that of LIBs; therefore, SIBs are promising next-generation alternatives. Recently, sodiated layer transition metal oxides, phosphates and organic compounds have been introduced as cathode materials for SIBs. Simultaneously, recent developments have been facilitated by the use of select carbonaceous materials, transition metal oxides (or sulfides), and intermetallic and organic compounds as anodes for SIBs. Apart from electrode materials, suitable electrolytes, additives, and binders are equally important for the development of practical SIBs. Despite developments in electrode materials and other components, there remain several challenges, including cell design and electrode balancing, in the application of sodium ion cells. In this article, we summarize and discuss current research on materials and propose future directions for SIBs. This will provide important insights into scientific and practical issues in the development of SIBs.
1. Introduction
Fossil fuels are the most widely used energy resource worldwide. Risks related to resource depletion, environmental pollution, and political unrest with regard to fossil fuel production have led to the rapid emergence of a variety of intermittent renewable and cleaner energy sources such as wind, solar and wave. In order to integrate these renewable energies into the electrical grid, a large-scale energy storage system (ESS) is vital to peak shift operation.1 Among various energy storage technologies, using an electrochemical secondary battery is a promising method for large-scale storage of electricity due to its flexibility, high energy conversion efficiency, and simple maintenance.1,2 LIBs, which have become common power sources in the portable electronic market since their first commercialization by Sony in the early 1990s,3 are the primary candidates for ESSs. The introduction of LIBs into the automotive market as the battery of choice for powering hybrid electric vehicles (HEVs), plug-in hybrid electric vehicles (PHEVs) and electric vehicles (EVs) could reduce dependence on fossil fuels. Lithium, the primary ingredient in LIBs, is non-uniformly distributed within the Earth’s crust. As a result, the Andean states have been dubbed the ‘new Middle-East’.4 However, the increasing demand for lithium associated with these new and large-scale applications is expected to skyrocket the price of lithium, affecting reserves as well, as it is not a naturally abundant element. Based on the calculations, overall global Li consumption in 2008 was nearly 21 280 tons; hence, present mineable resources could be sustained for approximately 65 years at most at an average growth rate of 5% per year,2,5 making the implementation of the above-mentioned applications difficult and very costly.
Sodium, the fourth most abundant element on earth, has a seemingly unlimited distribution.6 Supplies of sodium-containing precursors are vast, with 23 billion tons of soda ash located in the United States alone. The abundance of resources and the much lower cost of trona (about $135–165 per ton), from which sodium carbonate is produced, compared to lithium carbonate (about $5000 per ton in 2010), provide a compelling rationale for the development of SIBs to be used as alternatives to LIBs.7,8 Because an alternative to lithium is needed to realize large-scale applications, SIBs have attracted considerable research attention in recent years. SIBs were initially studied when the development of LIBs began in the 1970s and 1980s, but due to rapid advances in the development and success of commercial applications of LIBs, SIBs were largely abandoned.9–15 Moreover, during those years, the overall quality of materials, electrolytes and glove boxes was insufficient for handling sodium, making it difficult to observe electrode performance. In the 1980s, prior to the commercialization of LIBS, a few US and Japanese companies developed SIBs in full cell configurations where a sodium-lead alloy composite and a P2-type NaxCoO2 were used as the anode and cathode, respectively. Despite the remarkable cyclability over 300 cycles, the average discharge voltages were lower than 3.0 V, which did not attract much attention against carbon//LiCoO2 cells exhibiting an average discharge voltage of 3.7 V.16–18 The battery components and the electrical storage mechanism of SIBs and LIBs are basically the same except for their ion carriers. In terms of cathode materials, the intercalation chemistry of sodium is very similar to that of lithium, making it possible to use similar compounds for both systems. However, there are some obvious differences between these systems. Na+ ions (1.02 Å) are larger compared to Li+ ions (0.76 Å), which affects the phase stability, transport properties, and interphase formation.9 Sodium is also heavier than lithium (23 g mol−1 compared to 6.9 g mol−1) and has a higher standard electrode potential (−2.71 V vs. SHE as compared to −3.02 V vs. SHE for lithium); thus, SIBs will always fall short in terms of energy density. However, the weight of cyclable Li or Na is a small fraction of the mass of the components, and the capacity is determined primarily by the characteristics of the host structures that serve as electrodes. Hence, in principle, there should be no energy density consequences of the transition from LIBs to SIBs.7 In addition, aluminum undergoes alloy reaction with lithium below 0.1 V vs. Li/Li+, which indicates that aluminum is available as a current collector for anodes in sodium cells. Therefore, aluminum is a cost-effective alternative to copper as an anode current collector for SIBs.
Various cathode materials for SIBs have been reported; for instance, layer and tunnel type transition metal oxides, transition metal sulfides and fluorides, oxyanionic compounds, Prussian blue analogues and polymers. However, the search for an anode with appropriate Na voltage storage, a large reversible capacity, and high structural stability remains an obstacle to development of SIBs. Graphite, which is a common anode material in LIBs, has a moderate Li storage capacity (∼350 mA h g−1) at approximately 0.1 V vs. Li/Li+.2 Recent studies have demonstrated that graphite does not properly intercalate sodium ions.19,20 Non-graphitic anodes, which consist largely of various carbonaceous materials such as carbon black21 and pitch-based carbon-fibers,22 allow insertion of sodium ions. Hard carbons, which are synthesized at high temperatures from carbon-based precursors, have been comprehensively modeled,23,24 characterized,25 and thermally tested26 in Na cells. These non-graphitic carbonaceous materials are considered to be the “first-generation” anodes of choice for SIB systems. SIBs are not fabricated with sodium metal due to dendrite formation, high reactivity, and an unstable passivation layer in the most organic electrolytes at room temperature. The high reactivity of metallic sodium with organic electrolyte solvents and dendrite formation during Na metal deposition are even more problematic than they are in Li metal anodes. The low melting point of sodium at 97.7 °C also presents a safety hazard for devices using Na metal electrodes at ambient temperature.27 Thus, it is important to use a true Na-ion system, where Na ions are exchanged between cathodes and anodes in a ‘rocking-chair’ format.
A new type of electrolyte for SIBs is needed, as the use of organic liquid electrolytes raises practicality and safety issues. The most common electrolyte formulations for SIBs are NaClO4 or NaPF6 salts in carbonate ester solvents, particularly propylene carbonate (PC). Metallic sodium anodes corrode continuously in the presence of these organic electrolytes, rather than forming a stable solid electrolyte interface (SEI). According to XPS and TOF-SIMS analyses performed by Komaba et al.,27 when NaPF6 is used as the electrolyte salt, the SEI film on hard carbon is predominantly an inorganic salt that contains precipitated species such as NaF on the surface.7 Palacin and colleagues28 found that NaClO4 and NaPF6 in an EC:PC solvent mixture represent the best electrolyte for a hard carbon anode. Developing aqueous electrolytes instead of organic electrolytes could be essential to the success of SIBs. Recently, an aqueous rechargeable battery with Na2NiFe(CN)6 and NaTi2(PO4) as the cathode and anode, respectively, demonstrated a good rate and cycle life with a theoretical energy density of 42.5 W h kg−1.29 Thus, it is possible to achieve higher energy density by selecting the appropriate electrode material. Nevertheless, an aqueous electrolyte system is more complicated than an organic system because of the (1) elimination of residual O2 from the electrolyte, (2) maintenance of electrode stability in the aqueous electrolyte, (3) inhibition of H3O+ co-intercalation into the electrode and (4) efficiency of the internal consumption of O2 and H2 produced from the cathode and anode sides when overcharged, overdischarged or improperly operated in a closed aqueous battery system. All of these issues are important for practical applications of aqueous battery systems.30,31
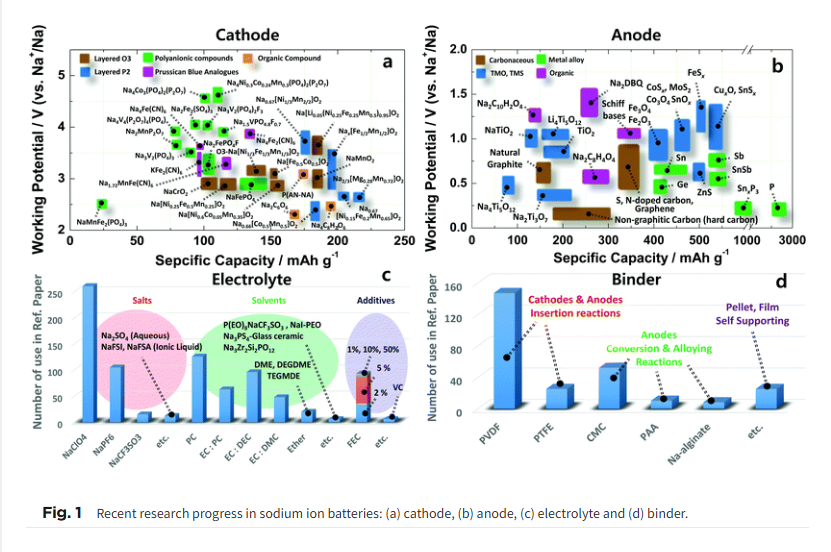
Scheme 1 and Fig. 1 illustrate a schematic SIB image that can adopt several representative candidate materials such as cathode and anode materials, electrolytes, separators, and binders that are discussed in the present paper. Most studies of SIBs explored the electrochemical performance of new electrodes and materials used with Na metal in half-cells, as this field is still in its early stages, and it is thus difficult to create full cells. In that regard, discussion and justification of SIBs are complicated compared to those of LIBs. In addition, experimental conditions such as the purity of Ar gas, electrolyte quality, and the glove box can influence the performance of SIB cells. Hence, comparison of battery performance between studies can be challenging.18
2. Cathode materials
Similar to LIBs, highly reversible cathode materials based on the intercalation reaction, which involves interstitial introduction of a guest species (Na+ in the present context), are needed for high capacity and good cyclability of SIBs. These electrode materials are mainly categorized into oxides, polyanions such as phosphates, pyrophosphates, fluorosulfates, oxychlorides, and NASICON (Na super ionic conductor) types, and organic compounds, which are mentioned in detail in Sections 2 and 3. These cathode materials exhibit a minimal structural change with intercalation, which ensures a reversible intercalation reaction that affects the cycle life. However, continuous structural evolution is inevitable during Na+ ion intercalation into the host structure interaction because of the large Na+ ion size (coordination number 6: 1.02 Å) relative to that of Li+ (coordination number 6: 0.76 Å). Also, sodiated transition metal materials are highly hygroscopic, even with brief exposure to air,32 and caution is necessary to avoid hydration of the material, particularly the surface, which results in formation of NaOH that degrades electrode performance due to its insulating properties. Thus, the preparation of sodiated cathode materials and batteries requires meticulous handling and moisture-free conditions.
Read more: Sodium-ion batteries: present and future
If you do battery research or battery materials research, you might be interested in these products: NEWARE
